Informed Consent Is Required in Which of the Following Situations
Requirements for informed consent. In obtaining and documenting informed consent the investigator should comply with the applicable regulatory requirement s and should adhere to GCP and to the ethical principles that have their origin in the Declaration of Helsinki.

Pin On Chamberlain College Of Nursing
Some e xamples of situations when written informed consent is either required under the Code or may be required by your health care provider include.

. Waives the requirement to document informed consent ie to obtain a signature. It informs the participants about the trial and lets them make educated decisions about taking part in the study. B The purpose of the trial.
Informed consent for research or clinical trials is also required. The Common Rule the IRB may approve an informed consent process that. In the case of a HIV test consent should preferably be written although consent may be implied.
Link is external For all medication and treatment unless court-ordered link is external Informed consent for medications. US federal regulations require a full detailed explanation of the study and its potential risks. When you are having surgery an operation or other procedure.
Waives the requirement to obtain informed consent or. Consent The following bullet points summarise BHBIAs guidance on Consent and are drawn from Legal Grounds for Data Processing see Appendix 1 on page 6 for the full details. Under the Federal Policy for the Protection of Human Subjects aka.
2 the IRB finds and documents that informed consent can be waived 45 CFR 46116 c or d. Informed consent is a legal and medical term that refers to a patients right to know about the risks involved with a course of treatment or medical procedure before he or she decides in favor of a recommended treatment plan or medical procedure. 1 disclosure of information 2 competency of the patient or surrogate to make a decision and 3 voluntary nature of the decision.
The patient must be capable of consenting. Broad consent may be obtained in lieu of informed consent obtained in accordance with paragraphs b and c of this section only with respect to the storage. Explains confidentiality O b.
Under state law a patient must provide written informed consent in the following situations. The following are elements of informed consent. The HHS regulations at 45 CFR part 46 for the protection of human subjects in research require that an investigator obtain the legally effective informed consent of the subject or the subjects legally authorized representative unless 1 the research is exempt under 45 CFR 46101 b.
To perform labor which is of financial benefit to the facility. In a doctors office hospital or other medical setting healthcare providers are required to obtain informed medical consent before treating a patient. Informed Consent of Trial Subjects.
-patient must be given sufficient information about the treatment and alternatives. 4810 Both the informed consent discussion and the written informed consent form and any other written information to be provided to subjects should include explanations of the following. Which one of the following is a reason for revoking a physicians license.
The type of consent in which the client is told of the complete nature and treatment of a proposed procedure as well as consequences of not having the procedure is. The privilege of confidential communications is controlled by the health care provider. The study hypothesis O C.
A short form written consent document stating that the elements of informed consent required by 5025 have been presented orally to the subject. Consent must be given by a clear affirmative action Consent must be freely given clear obvious and informed. You sign up for a psych study on campus to make a little extra money for weekend fun.
General requirements for informed consent whether written or oral are set forth in this paragraph and apply to consent obtained in accordance with the requirements set forth in paragraphs b through d of this section. Consent must not conflict with good morals or the Constitution. Informed consent can apply in emergency and nonemergency situations.
That the only record linking the subject and the research would be the informed consent form and the principal risk would be potential harm resulting from a breach of confidentiality. The Board can waive the requirement for the investigator to obtain a signed informed consent form for some or all subjects if any of the following apply. 51531-51533 the Secretary HHS announced under Section 46101i a waiver of the applicability of the 45 CFR Part 46 requirement for obtaining and documenting informed consent for a strictly limited class of research involving research activities that may be carried out in human subjects who are in need of emergency.
A That the trial involves research. When a doctor does not provide information about possible risks and the patients is injured as a result of the procedure or. Description of study procedures QUESTION 7 Yuusui Completion Status.
2829 However in most other situations in the ED informed consent is presumed for the patient. However obtaining informed consent may not be possible in emergency situations. Issues of facility safety such as floor surfaces stairways windows etc are generally subject to med-.
Prior to the beginning of the trial the investigator should have the IRBIECs written approvalfavourable. Valid informed consent for research must include three major elements. The American Medical Association explains that in some scenarios people who are severely injured or in dire states of health cannot be informed about medical treatments and are unable to communicate with physicians nurses or a trauma team.
-patient must be competent to make an informed decision. Risks and benefits O d. C The trial treatments and the probability for random assignment to each treatment.
In other words the patient is presumed to have consented to any and all relevant. CHAPTER 2 PART 2 OF 3. The general rule with regard to informed consent in an emergency circumstance is that the standard informed consent rule still applies to cogent conscious adults who require treatment.
-patient must voluntarily give consent. Alters some or all of the elements of informed consent or. The privilege of confidential communications is controlled by the patient.
On October 2 1996 Federal Register Vol. Consent must be voluntary and without constraint. QUESTION 6 Which of the following is NOT a required element of informed consent.
In general informed medical consent means advising the patient of reasons the treatment is needed the benefits of having it done the risks of harm that may occur and any alternative treatments that may be.

What Is Informed Consent In Healthcare 4 Principles Important Laws
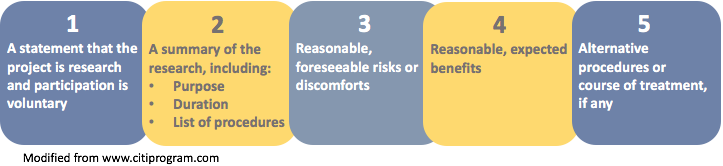
Informed Consent Guidelines Templates Research Ethics Compliance
No comments for "Informed Consent Is Required in Which of the Following Situations"
Post a Comment